Lesson Plan
Grow it Now, Drive it Later?
Grade Level
Purpose
Students will discover potential careers in agriculture with a focus on the growing field of biofuel development. Grades 6-8
Estimated Time
Materials Needed
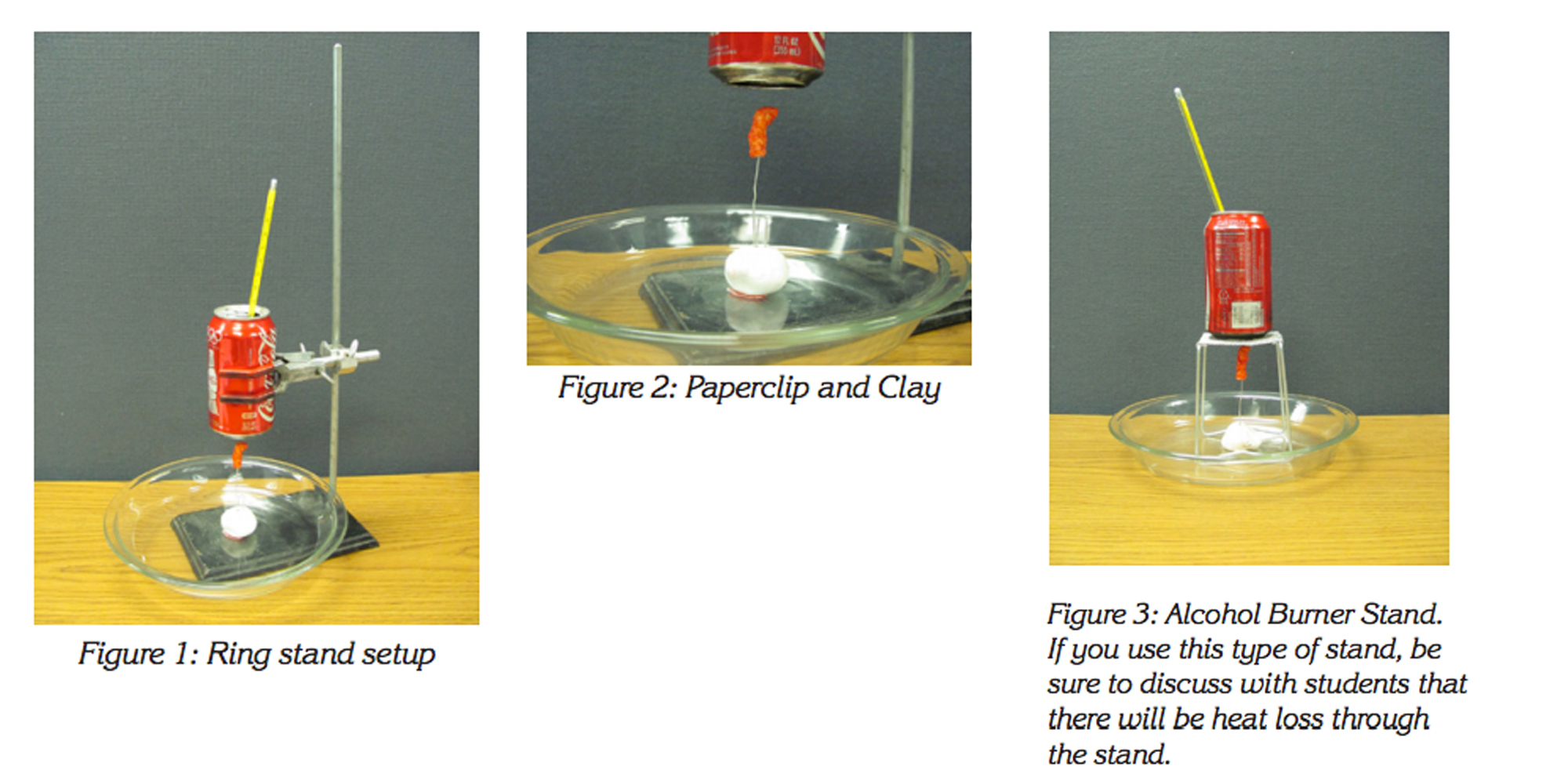
- Ring stand or other type of can support, see Figure 1
- Aluminum pop can
- Pyrex pie plate or dish (optional)
- Flamin’ Hot Cheetos® (soybean oil)
- Peanuts, or dried algae
- Modeling clay to hold paperclip
- Large paperclip
- Graduated cylinder or beaker
- Celsius thermometer
- Lighter or stick match
- Water
- Projected visual of Calorie Calculation
Vocabulary
algaculture: farming a species of algae
algae: a simple nonflowering plant of a large group that includes the seaweeds and many single-celled forms; contain chlorophyll but lack true stems, roots, leaves, and vascular tissue
biodiesel: a fuel made from vegetable oils or animal fats
ethanol: a fuel produced by fermentation of products high in starch, such as corn
petroleum: a naturally occurring liquid found in geological formations beneath the earth's surface which is often refined into fuel
Background Agricultural Connections
Plants take in light energy from the sun and turn it into sugars. They store the sugars in their roots, leaves, stems, flowers, and seeds. The energy in the sugars makes them grow. When people or animals eat food made from plants like soybeans, corn, and wheat, the sugars (or carbohydrates) give them energy. In addition to sugars, the seeds of plants also contain protein and fats (or oils).
The sugars in plants may be distilled to make ethanol, a fuel source for combustion engines. The seeds of plants have long been used as an energy or fuel source for diesel engines. Peanuts and soybeans have commonly been used to make biodiesel, and several other seeds high in oil have been used as well.
 Soybeans belong to the legume family—plants that produce beans in pods. Legumes also take in nitrogen from the air and release it into the soil. Nitrogen is important for good soil and healthy crops. One soybean plant produces about 70 soybean pods with two to four beans in each pod. The seeds are about the size of peas and may be yellow, green, brown, black, or speckled.
Soybeans belong to the legume family—plants that produce beans in pods. Legumes also take in nitrogen from the air and release it into the soil. Nitrogen is important for good soil and healthy crops. One soybean plant produces about 70 soybean pods with two to four beans in each pod. The seeds are about the size of peas and may be yellow, green, brown, black, or speckled.
Soybeans were first domesticated in Asia, where farmers have grown them for more than 5,000 years. Today soybeans are still an important crop in China. They are used for food, fertilizer, animal feed, medicines, and oils.
Soybeans were first grown in the United States in the early 1800s. They were used as a source of food for humans and farm animals. During the Civil War, coffee beans were hard to get, so soybeans were roasted and used to make a coffee substitute. They were called coffee berries.
In 1904 George Washington Carver began studying soybeans. He discovered that soybeans are a valuable source of oil as well as protein. A 60-pound bushel of soybeans produces 48 pounds of soy protein, 11 pounds of soy oil, and 1 pound of hulls (the coatings of the beans). Henry Ford used soy oil to make plastic parts for his cars.
Farmers harvest the soybeans, which can be eaten fresh in their pods, dried and roasted, or broken down into different forms. Most soybeans are harvested and removed from their pods by a machine called a combine and then sent to a processing plant where they are crushed, rolled into flakes, and mixed with a solvent to separate the oil and protein. Soybean oil and protein can be made into many kinds of animal and human foods, as well as products such as crayons, paint, glue, and plastics.
Soybeans can be blended (mixed) with regular diesel, which is a petroleum fuel. Many cities and school districts use a blend of 20 percent biodiesel and 80 percent diesel in their buses. This is called a B20 blend. Using B20 reduces pollution from the buses, making the air cleaner.
Most trucks, buses, and tractors in the United States use diesel fuel. Regular diesel fuel is made from petroleum, a nonrenewable energy source. Petroleum is a fossil fuel; it takes millions of years to form under the ground, so we can’t make more in a short time. When petroleum fuels are burned in vehicle engines, they can pollute the air. If they spill onto the soil or into the water, they can damage the environment for a long time. Petroleum fuels are toxic and should be handled carefully.
Biodiesel is a fuel made from vegetable oils or animal fats. It is usually made from soybean oil, but it can also be made from corn oil or used restaurant grease and oil. If it is made from restaurant oil, it can smell like French fries. Since biodiesel is made from plant and animal oils, it is a renewable fuel. We can grow more plants in a short time to make more biodiesel. However, with the demand for food and land resources increasing, some scientists are looking at a “plant-like” organism—algae—as a possible fuel source. High oil prices and the competing demands between foods and other biofuel sources have ignited interest in algaculture (farming algae) for making vegetable oil, biodiesel, and other biofuels using land that is not suitable for agriculture.
 Algae are not plants. They lack the structures that characterize land plants, such as leaves and roots. Nearly all algae have photosynthetic capability, and algae cells contain both sugars (carbohydrates) and oils (fats). Scientists are looking at efficient ways to grow algae for biofuels. Algae don’t need fertile soil and can grow or colonize in wastewater. Several types of algae grow well in salt water. Algae use sunlight and water and produce oil in their cells 50 times faster than regular crops. When this oil is extracted for biofuel, it can be used in a car and can be used to generate electricity. Among algal fuels’ attractive characteristics: they do not affect fresh water resources, they can be produced using ocean and wastewater, and they are biodegradable and relatively harmless to the environment if spilled.
Algae are not plants. They lack the structures that characterize land plants, such as leaves and roots. Nearly all algae have photosynthetic capability, and algae cells contain both sugars (carbohydrates) and oils (fats). Scientists are looking at efficient ways to grow algae for biofuels. Algae don’t need fertile soil and can grow or colonize in wastewater. Several types of algae grow well in salt water. Algae use sunlight and water and produce oil in their cells 50 times faster than regular crops. When this oil is extracted for biofuel, it can be used in a car and can be used to generate electricity. Among algal fuels’ attractive characteristics: they do not affect fresh water resources, they can be produced using ocean and wastewater, and they are biodegradable and relatively harmless to the environment if spilled.
How do you get oil out of soybeans? Soybeans are crushed and then a solvent called hexane is used to extract oil from the seed. If you crush soybeans in a mill and then boil the meal, the oil from the seeds will float on the surface of the water. How do you get the oil out of algae? It is similar to soybean oil extraction; you remove the oil from dried algae chemically with hexane or mechanically with an expeller press or ultrasound. How much energy is in oil? Conduct the following activity to measure the calories in vegetable oil.
Engage
- Ask your students this series of questions to help them begin thinking about fuel:
- "What is required to have in our cars, trucks, and other vehicles for them to run?" (Fuel)
- "Where does most fuel come from?" (Most fuels have a petroleum base. It is pumped from deep within the ground and then refined)
- "Are petroleum fuels a renewable or non-renewable resource?" (non-renewable)
- "Are there any types of fuel that are renewable?" (Yes, biofuels. Ask further questions and discuss the topic of biofuels, ethanol, biodiesel, etc. to determine your student's prior knowledge on the topic.)
Explore and Explain
- Use the ring stand to hold the aluminum can as pictured in Figure 1.
- Place a handful of modeling clay on the bottom of the ring stand or in a Pyrex dish at the bottom of the ring stand.
- Open one side of a large paperclip and place the bent portion into the clay as shown in Figure 2.
- Impale (spear) half a Cheeto onto the paper clip. (If you use more than half a Cheeto, you may set off a smoke detector.)
- Move the can to within two inches or five centimeters of the impaled Cheeto.
- Measure and add 100 milliliters of water to the can.
- Record the temperature of the water on the board. To find out how many calories are stored in the soybean oil in half a Cheeto, light the Cheeto and burn it to see how much energy is produced as noted by the rise in water temperature. See the Calorie Calculation activity sheet.
- Record the water temperature as soon as the flame burns out.
- Calculate the calories using the formula on the Calorie Calculation activity sheet.
- Repeat the procedure with a peanut or with algae.
- View algae oil videos from YouTube (search algae biofuel) or see the following:
How do you convert ml to grams?
One gram of water is always equal to 1 ml because that is its weight. For other substances, this is not necessarily true because different substances have different densities. For example, 1 ml of iron weighs about 7 grams; it is more dense than water.
1 ml of water = 1 gram
1 ml of iron = about 7 grams
Recommended Companion Resources
Author
Organization
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |