Lesson Plan
FoodMASTER Middle: Cheese
Grade Level
Purpose
Students will learn about the Law of Conservation of Mass by exploring environmental factors that can impact protein coagulation in milk (cheese-making process). By making qualitative and quantitative observations they will test three possible methods of making curds and whey. Grades 6-8
Estimated Time
Materials Needed
Lab:
- Acid Adventure student handout, 1 per student (Key)
- Maintaining Mass lab sheet, 1 per student (Key)
Teacher Demonstration (see lesson Procedures step 4)
- Safety goggles
- Apron (optional)
- 1 cup 2% milk
- 2 small clear plastic cups (labeled “curds” and “whey)
- 1 hot plate or double burner
- 1 cup for massing the milk
- 1 small saucepan
- 1 triple beam balance
- 1 whisk or metal spoon
- 1 black permanent marker
- 1 medium bowl
- 1 plastic spoon
- 1 small strainer (very thin mesh)
Student Lab Materials (per group of 4-5 students)
- Safety goggles
- Aprons (optional)
- 3 plastic spoons
- 1 small strainer (very thin mesh)
- 1 triple beam balance
- 4 Styrofoam cups
- 1 medium bowl
- 1 liquid measuring cup
- 1 black permanent marker
- 2% Milk
- 1 small cup containing 1 tbsp. of baking soda
- 1 small cup containing ½ cup of vinegar
Investigating Your Health Activity:
- Charming Cheese student handout, 1 per student
- Cheese Nutrition Fact Labels
- Students may obtain their own labels or use the examples provided.
Vocabulary
casein: a protein that makes up about 80% of milk
coagulation: changing a liquid to a soft semi-solid or solid mass
curd: clumps of casein (coagulated protein) that separates from the liquid when milk coagulates
protein: an essential nutrient responsible for building tissue, cells, and muscle
tare: an allowance made for the weight of a container when trying to determine the net weight of a substance
whey: liquid that drains from the curd of clotted milk; contains lactose, proteins, water-soluble vitamins, and some minerals
Did You Know?
- The familiar yellow color to cheese such as mild and sharp cheddar is the result of an artificial dye. All cheese is naturally white, just like milk.1
- There is an amino acid in casein milk protein called tyrosine. When tyrosine hits your brain it triggers mood-boosting neurotransmitters that improve attention, focus, and happy feelings.2
- The holes in swiss cheese are a byproduct of the bacteria used to make the cheese.3
Background Agricultural Connections
Milk, cheese, yogurt, and ice cream are all part of the dairy group. Drinking milk helps build strong bones. Unfortunately, most Americans do not drink or eat enough foods from the dairy group every day. For this reason, it is important to learn about the science and nutrition of dairy foods. In this lesson, students will explore milk by learning how cheese is made, the effect of pH on the formation of curds and whey, and the many health benefits associated with consuming dairy products.

The Cheese-Making Process
A lactic-acid producing bacterium is added to milk to begin the cheese making process. This allows the milk to separate into curds and whey. The curds are then cut into smaller pieces allowing the liquid portion, whey, to escape. Whey is the watery portion of milk that separates from the curds when cheese is being made. Heat is applied to the curds to speed up the separation of whey. After the whey has been separated, the curds are drained, stretched, salted, and pressed to form a more concentrated cheese.
To give each cheese its own unique properties, it is then cured (“aged”) or ripened to complete the process. Cheese that needs to be cured is not ready for consumption after being prepared.
Depending on the desired characteristics, the cheese is held for a certain amount of time, at a specific temperature, and under certain conditions. Most cheeses we consume are ripened, unless they are fresh, such as fresh mozzarella or ricotta. Fresh cheeses can be consumed right after they are made. Ripening is considered the changes that occur between the formation of curd and the development of the desired characteristics such as aroma (smell), flavor, texture, and composition.
Factors Affecting Curd Formation and Coagulation
There are many factors that contribute to the cheese making process. The composition of milk is considered the most important factor affecting the curd formation. More specifically, milk’s fat and protein concentration impact curd formation because curds are primarily coagulated casein (protein). If whole milk is used, fat globules are also entrapped in the curd.
The type of acid used and temperature of milk during coagulation can also impact curd formation. Coagulation is the breakdown and reformation of proteins by heat. The curd formed at this point is soft. For example, rennet is the general term for any enzyme used to make cheese. Rennet can be found in the lining of a calf’s stomach. Scientists have been able to duplicate an artificial version of rennet to be used in larger amounts.
The following factors influence the curdling properties of milk:
- Bacteria: Bacteria can be added when making cheese or yogurt, but can also develop as milk begins to sour.
- Acids: Acids are found in fruits, fruit juices, and some vegetables (e.g. tomatoes). The addition of an acid will result in the precipitation of casein, the most abundant milk protein. Acid produces a soft and spongy texture due to the decreased pH.
- Tannins: Tannins are found in coffee and tea. They will curdle milk in the presence of acid and heat because they readily bond with protein. For example, if you add slightly old (soured) milk to coffee, you may see curdles form. The acidity of the soured milk and heat cause the milk’s protein to bond with the tannins (and itself) to form curdles.
FoodMASTER Middle Lessons
FoodMASTER (Food, Math and Science Teaching Enhancement Resource) is a compilation of programs aimed at using food as a tool to teach mathematics and science. For more information see the Background & Introduction to FoodMASTER for Middle School. This lesson is one in a series of lessons designed for middle school:
Engage
- Ask students to brainstorm all of the varieties of cheese they are familiar with. Following the brainstorm, ask students what kind of characteristics differentiate these cheeses. Answers may include color, moisture, taste, softness, texture, etc.
- Ask students what they know about the cheese making process. Follow up by watching the video clip, How Its Made: Cheese.
Explore and Explain
Activity 1: Lab – Maintaining Mass
Teacher Preparation:
- Review information found in the Background Agricultural Connections section of the lesson, lesson Procedures, and the attached Essential Files.
- Prepare materials for each group. Note that baking soda and vinegar should be pre measured in cups.
- In the investigation below, you (the teacher) will be using a hot plate to demonstrate the curdling properties of milk proteins. Students will investigate the curdling properties of milk protein through non-heat methods.
- Timesaver: Review scale “taring” and instruct students to weigh and label empty containers before performing the lab. Practicing measurement is important for students. However, if time is a concern, students may be provided teacher-determined masses of 1 tbsp. of baking soda and 1/2 cup of vinegar, if desired. Additionally, milk may be poured into pitchers for easier access by students. If time is a limitation, use the demonstration video in the student pre-lab materials in place of the in-class teacher-led demonstration. Provide students with the mock data below after your class has viewed the video.
Procedures:
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Maintaining Mass lab sheet and the Acid Adventure handout.
- Ask students to read Acid Adventure.
- Before beginning the lab investigation:
- Require students to wash their hands.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances used as part of the lab investigation.
- For food safety reasons, DO NOT allow students to taste any casein or whey byproducts.
- Launch the lab by providing a demonstration for your students. The demonstration will show students how to find the mass of the coagulated protein, while revealing that milk will curdle after exposure to high heat. View the demonstration video for illustration and follow the steps below:
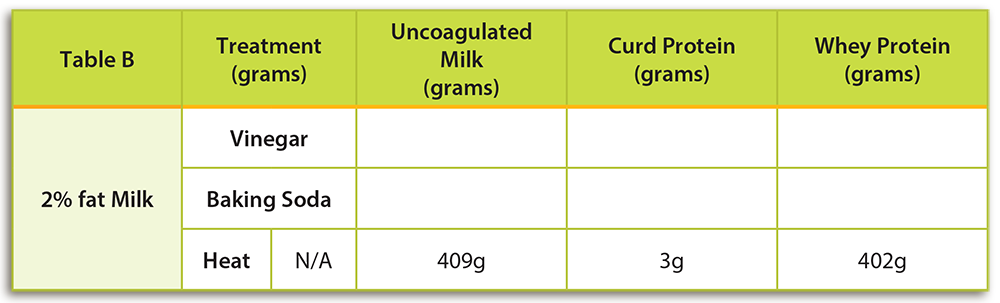
- Using a triple beam balance, mass 1 cup of 2% milk. Remember to mass the empty cup first. Record the mass in a location visible to the class to help demonstrate conservation of mass. Have students record the mass of the milk only in Table B under “Un-coagulated Milk” for the “Heat” treatment. Hint: This is a good time to review rules for massing liquids and powdered solids on a balance.
- At high heat, quickly heat the milk to a boil in a small saucepan. Use a whisk or metal spoon to stir the milk as it heats to prevent the milk from burning.
- Once the milk has begun boiling, continue to let it boil for 5 minutes. Be careful to continue whisking the milk as it heats. If the milk gets too hot and is not stirred, it will boil over.
- To determine the quantity of coagulated proteins you will strain and determine the masses of both the coagulated proteins and the liquid remaining.
- Place a clean, clear small cup on your scale. A clear cup is preferred so that students can easily see any coagulated proteins. Determine the mass of the cup.
- Next, place a strainer over a medium bowl and pour the heated skim milk into the strainer. If present, the strainer will catch any coagulated proteins. Using a plastic spoon, scrape the coagulated proteins out of the strainer with a plastic spoon and place them in the clean cup measured in STEP 1.
- Place the clear cup containing the milk proteins that you just scraped from the strainer on your balance. You may need to scrape some coagulated protein from your pot too. The coagulated proteins are called curd proteins. Subtract the mass of the cup from the mass of the cup and curds. Have students record the mass of the curd proteins in Table B under the column labeled “Curd Protein.”
- The left over fluid in your bowl is called whey protein. Determine the mass of a second clean, clear cup. Pour the liquid whey protein into a clear cup. Subtract the mass of the cup from the mass of the cup and whey. Have students record the mass of the whey in Table B under the column labeled “Whey Protein.”
- Begin the student portion of the lab investigation by asking students to observe and make a prediction about treatments that may cause milk to curdle (see the bottom of page 1 of their lab sheet). Based on the teacher demonstration completed in the previous step, students should already know that heat would cause milk to curdle. After the lab, students should recognize the following reactions:
- Heat: Students should observe clear curdling. When milk is heated, a precipitate will form. The heat-sensitive coagulated proteins are mostly whey and can generally be observed on the bottom and sides of the cooking pan. Curd proteins will not form as readily when milk is heated. Milk may have to be heated to higher temperatures (212°F, 100°C) for longer time periods to observe curd formation.
- Vinegar: Students should observe clear curdling. When an acid is added to milk it causes the normal pH (6.5 – 6.7) to fall. A lower pH causes the milk proteins to destabilize. Curd proteins will precipitate and be observable. Whey proteins are not as sensitive to acid as curd proteins and will remain in a colloidal suspension (fluid state).
- Baking Soda: No curdling should be observed. The addition of baking soda will result in an increase in pH. Milk proteins will remain stable.
- Use the Maintaining Mass video to demonstrate the steps that students will now complete in their groups.
- Allow students to work in small groups on the Maintaining Mass lab sheet to complete the lab and respond to questions.
- Allow students to share data between groups. Students should seek to complete their data tables with information for the treatment they were not originally assigned.
- Follow-up with a class discussion about the impact of various environments on proteins (e.g. heat, acidic, basic) and the relevance to the cheese-making process.
Activity 2: Investigating Your Health – Charming Cheese:
- Give each student one copy of the Charming Cheese student handout. This assignment can be completed in class or as a homework assignment.
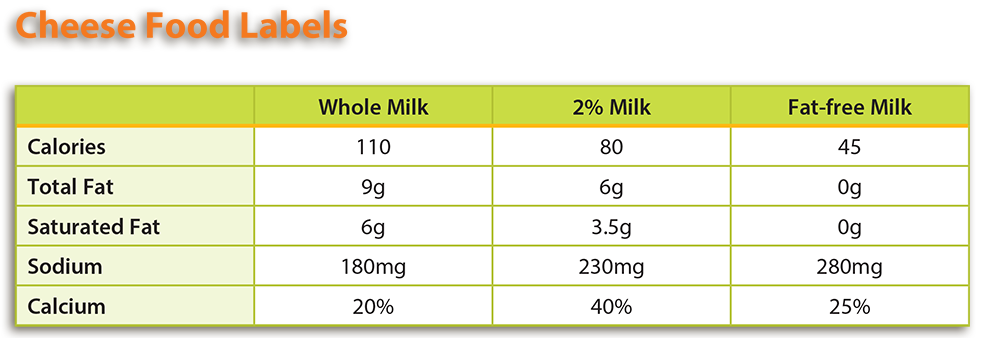
- Using the information found in the Background Agricultural Connections section of the lesson and the Acid Adventure handout, students should examine the food labels of different types of cheese and compare them based on fat and calcium content. Students can find food labels in the grocery store, USDA’s FoodData Central, or by using the examples provided.
- Note: If you choose to use the provided cheese food labels, see the attached Teacher Key for answers. Answers to questions based on other food labels will vary.
- To standardize serving size across nutrition labels, students will need to convert each serving size into the same value (cups, tablespoons, etc.). Students will then need to convert all fractions to a decimal. Finding the largest decimal, the students will then divide it by one of the others. Multiply each number in the nutrition facts label using this answer. For example: 3/4 = .75, .75 ÷ .5 = 1.5. Repeat these steps with the other labels. Please see the example below.
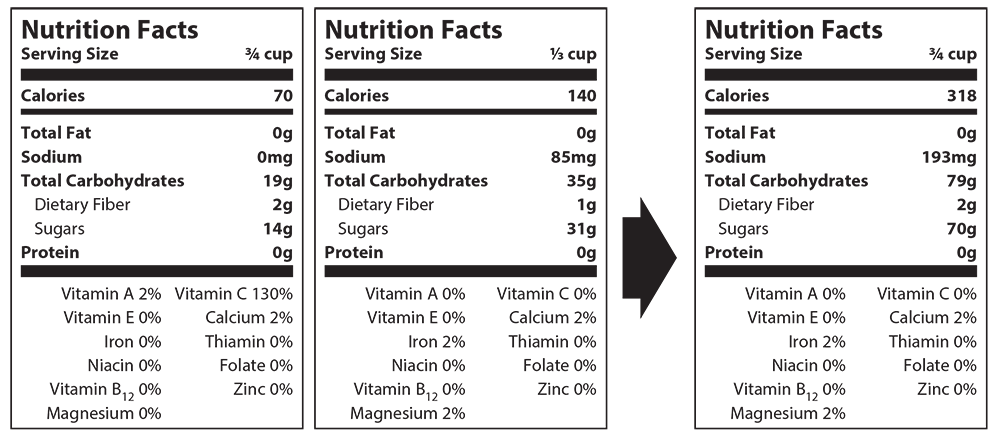
- If completed in-class, allow students to work in small groups on the Charming Cheese student handout and respond to questions.
- Conversion Factors:
- 3/4 = 0.75
- 1/3 = 0.33
- 1/2 = 0.5
- 1/3 cup x CF: 0.33 x 2.27 = 0.75
- Calories: 140kcal x 2.27 = 193mg
- Carbohydrate: 35g x 2.27 = 79g
- Dietary Fiber: 1g x 2.27 = 2g
- Sugars: 31g x 2.27 = 70g
- Conversion Factors:
Elaborate
-
Bring in a variety of cheese for your students to taste test.
-
Discuss the water activity properties of different cheese types. Which type of cheese will mold the fastest? (See FoodMASTER Middle: Food Safety)
-
Have students research the role of rennet in the making of cheese.
Sources
- https://www.cheeserank.com/post/cheese-facts-you-didnt-know
- https://www.cheeserank.com/post/cheese-mental-health
- http://www.todayifoundout.com/index.php/2010/11/why-swiss-cheese-has-holes-in-it/
Acknowledgements
This lesson was partnered with East Carolina University. The FoodMASTER program was supported by the Science Education Partnership Award (SEPA) which is funded from the National Center for Research Resources, a component of the National Institutes of Health.
- Primary Authors:
- Virginia Stage, PhD, RDN, LDN
- Mary White
- Ashley Roseno, MAEd, MS, RDN, LDN
- Melani W. Duffrin, PhD, RDN, LDN
- Graphic Design: Cara Cairns Design, LLC
Recommended Companion Resources
Author
Organization
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |