 Relevancy and Engagement
agclassroom.org/in/
Relevancy and Engagement
agclassroom.org/in/
Lesson Plan
Milk: The Scoop on Chemical and Physical Changes
Grade Level
Purpose
In this lesson students apply their knowledge of physical science to dairy products to determine if the changes that take place when turning milk into cheese, butter, yogurt, ice cream, whip cream and other dairy products, is a physical or chemical change. Grades 9-12
Estimated Time
Materials Needed
- Dairy Products-Chemical or Physical Change handout, 1 copy per class
- Internet access for each group of students
- Milk Products graphic
- The Milk Pasteurization Process infographic
Vocabulary
chemical change: a change that results in the formation of a new chemical substance through the making or breaking of bonds between atoms
physical change: a change in a substance that does not alter its chemical identity, including changes in shape, physical state, size, or temperature; this type of change is usually reversible
Did You Know?
- It takes approximately 2 and 1/2 gallons of milk to make 1 pound of butter.2
- It takes approximately 1 gallon of milk to make 1 pound of cheese.2
- The average cow in the United States produces 6-8 gallons of milk per day.3
Background Agricultural Connections
Milk & Food Processing
Processing is a term used to describe steps that are taken after the product leaves the farm, and before it is ready for retail sale. Many foods undergo various food processing procedures. Examples include curing Pork to make ham and bacon, turning apples into applesauce or cider, and squeezing oranges for orange juice. Food processing is a benefit to consumers. Food processing provides a greater variety of food to eat as well as food that is safer for human consumption. Without food processing our diets would consist of whole, raw foods exactly as they were produced on the farm and only in the season or shelf life of the food item. In addition, without food processing, consumers are at a greater risk of food-born illnesses.
Milk undergoes processing after it leaves the dairy farm. The fluid milk we drink and the milk that is used to make other dairy products such as ice cream, yogurt, and cheese is typically produced by cows on a dairy farm. In the United States, goats only provide a very small portion of milk for specialty markets. Milk leaves the dairy farm and must be processed prior to being sold to consumers. Fluid milk in the United States is pasteurized, homogenized, fortified, and standardized before it is sold to consumers.
- Pasteurization is a process originally created by Louis Pasteur in the mid 1800's. During pasteurization, milk (or another liquid) is heated to destroy microorganisms. As pasteurization practices became more common and eventually mandatory, milkborne diseases such as typhoid fever, scarlet fever, diphtheria, and diarrheal diseases were virtually eliminated. These diseases were especially devastating to young children and infants. Today, each state administers its own laws as to whether milk can be sold without pasteurization.
- Homogenization breaks up or shears the fat molecules (cream) in milk so that they are the same size and density of the rest of the milk. This results in a uniform texture and stops the cream from separating and rising to the top of the milk.
- Vitamin Fortification. Fluid milk in the United States is often fortified with Vitamin A and D. Vitamins A and D are found naturally in milk, however, they are fat-soluble vitamins. Milk with reduced fat levels (2%, 1% or skim) does not contain adequate levels of vitamin A without fortification. Vitamin D is critical to calcium absorption. Adequate calcium in our diet for healthy bones and teeth is only valuable when accompanied with vitamin D.
- Standardization. An important component of milk is fat. The percentage of fat found in milk depends on the species and breed of the animal that produced it. Other factors such as the animal's nutrition and the stage of lactation affect fat content. Most cattle produce milk with 3-5% milk fat. Consumers desire milk that is consistent in flavor and texture each time they purchase it. Consumers also desire lower fat milk products. Standardization is the process of removing fat from milk and adding it back to achieve the desired fat content. Whole milk (3.25%), 2%, 1% and skim (<.1%) milk are the result.
Physical & Chemical Changes
Once milk is pasteurized and homogenized it can be used to make many different food products. Each process requires milk to undergo a physical or a chemical change.
Physical changes can occur without altering the chemical composition of a substance. Physical changes can include changing the color, shape, state of matter, or volume of a substance. With many physical changes, the process can be reversed and return the product to it's original state. The following dairy products undergo a physical change:
- Butter: Butter is made using the cream (fat) found in milk. Through a process called churning, the fat globules in the cream separate from the buttermilk (remaining liquid) and make butter.
- Whip Cream: Whipping cream is made using the cream (fat) found in milk. When the cream is vigorously mixed or shaken the fat molecules begin clumping together thickening the once liquid cream into the solid state of whipping cream.
- Powdered Milk: Powdered milk is made by first concentrating milk until it is 50% solids. The concentrated milk is then heated. The water in the milk evaporates leaving a powder which can be stored and reconstituted later.
- Sweetened Condensed Milk: To make sweetened condense milk, milk is heated to evaporate water from the milk. This makes the milk thicker and more dense. Sugar is then added to sweeten the condensed milk. The sugar gives it the syrup-like consistency.
- Ice Cream: Ice cream is made first by mixing milk, cream, and sugar together. The mixture is then frozen into ice cream.
Chemical changes occur when bonds between atoms are made or broken. A new chemical substance is formed as a result and the process is permanent. The following dairy products undergo a chemical change:
- Cheese: Cheese is made when an enzyme called rennet is added to milk. The rennet causes a chemical reaction where the milk solids curdle, separating themselves from the whey, or liquid portion of the milk.
- Yogurt: Yogurt is made by adding a specific bacteria to heated milk. The milk is then incubated at a specific temperature for a period of time. The bacteria converts the lactose, or milk sugar, to lactic acid. This process thickens the milk and gives it the tangy taste.
- Sour Cream: Sour cream is made by adding a specific kind of lactic acid bacteria to cream. The addition of the lactic acid sours and thickens the cream.
- Cottage Cheese: Cottage cheese is made by adding an enzyme to milk causing it to curdle. Unlike hard cheeses, the cheese curds remain loose and are mixed with the whey (liquid portion) to create cottage cheese.
- Buttermilk: Buttermilk is made by adding a lactic acid bacteria to milk, giving it its characteristically sour taste.
In summary, physical changes occur in dairy products when water is simply evaporated from milk (powdered milk and sweetened condensed milk), when churning or whipping causes the fat molecules to stick together (butter and whip cream), or when a sugar is added (ice cream and sweetened condensed milk). Chemical changes occur when bacteria, acids, or enzymes are added to milk changing its taste, texture, or consistency.
Engage
- In this lesson students will be using milk processing to learn about science and chemistry. First, help them understand exactly what milk is. Ask your students, "What is milk?" Accept most reasonable answers and use further questions to guide students to discover and recall from prior knowledge the following facts about milk:
- "Where does milk come from?" (Milk is produced by all mammals after they give birth. Although all mammals produce milk, cows produce it most efficiently to make it available for human consumption.)
- "What is milk made of?" (Milk is composed mostly of water [approximately 87%]. It also contains other solids such as fat, various proteins, carbohydrates [lactose], vitamins, and minerals.)
- In this lesson students will:
- Describe the history and benefits of milk processing; and
- identify the physical and chemical changes that transform milk into various dairy products.
Explore and Explain
Activity 1: History of Milk Processing
- Ask your students, What does the term 'food processing' mean? Allow students to use context clues and their background knowledge to define the term. Explain to students that many of the food products we eat must be processed in some way. Food processing takes place after food leaves the farm and before a consumer purchases it. Give examples such as pork being cured to make ham or bacon, apples being made into applesauce or apple juice, etc.
- Ask your students, What happens to milk after it leaves the dairy farm and before it is sold as milk, cheese, or yogurt in the grocery store?
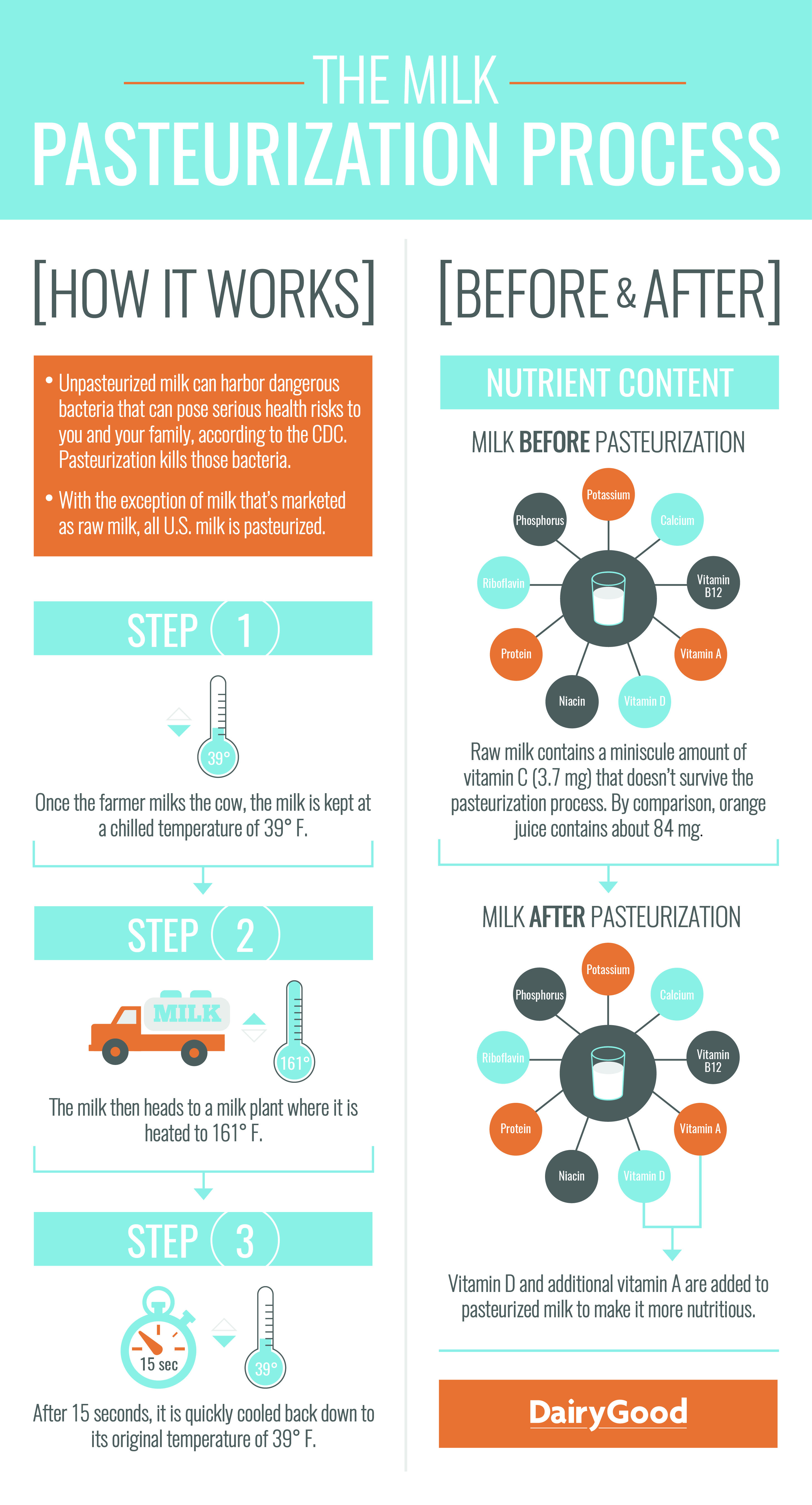
- Use the infographic, The Milk Pasteurization Process provided by the National Dairy Council to explain the process of milk pasteurization. Project the image for your class to see and follow the pasteurization steps listed on the left side of the graphic.
- Once your students have learned the basic steps in milk pasteurization, ask them to use their prior knowledge by asking them the question, Do you think milk changes in any way when it is pasteurized?
- After students have thought about the question and provided possible answers, review the right side of the infographic. Compare the nutritional contents of milk before and after pasteurization. Pasteurization has proven effective in minimizing bacteria that causes illness. This process is accomplished without significantly altering the physical or chemical nature of milk.
- Transition to the next activity by pointing out that while pasteurization does not significantly change the physical or chemical nature of milk, other processes do and they can be for our benefit.
| Help students draw a connection between science and our food supply (agriculture). Science plays a vital role in agriculture as our food is produced on the farm and processed (pasteurized) for our use. |
Activity 2: Chemical and Physical Changes to Make Dairy Products
- Write the words, "Chemical Change" on one side of your board and the words, "Physical Change" on the opposite side.
- Teach your students the difference between a chemical and physical change. Use the definition found in the vocabulary section of this lesson to define the difference between a physical and chemical change. List examples of each type of change to help students begin visualizing and understanding the principle.
- Examples of Physical Changes: Melting butter, water evaporating from a glass, molding clay into a new shape, etc.
- Examples of Chemical Changes: Rust forming on a nail, Baking a cookie, a piece of jewelry tarnishing, etc.
- Ask your students to brainstorm as many dairy products as they can think of. Make a list on the board. Although we drink fluid milk, the majority of milk produced is actually used to make dairy products such as yogurt, cheese, butter, ice cream, etc. How many dairy products can your students name?
- Using the graphic below, ask your students if it is a physical or chemical change that takes place when milk is made into various dairy products. To answer this question, your students must know a little more about how dairy products are made.
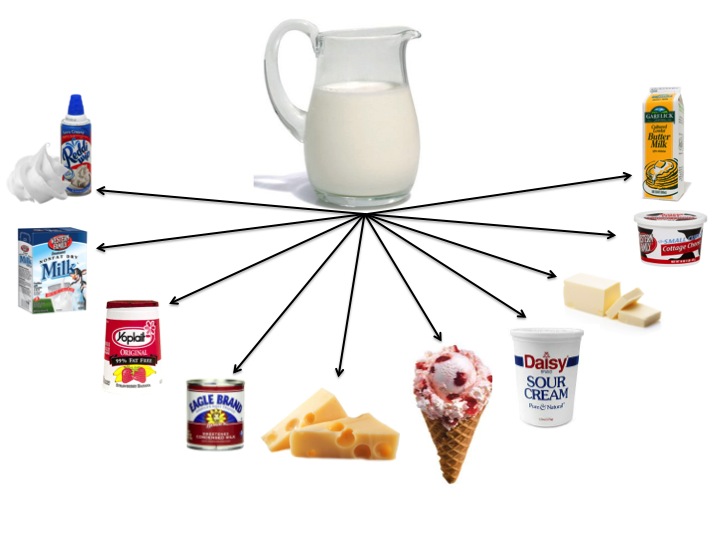
- Give each group 1 sheet of paper from the, Dairy Products-Chemical or Physical Change document. Each group will research the process of making a single dairy product. The students will learn how the dairy product is made and determine if it is a physical or chemical change that takes place.
- Provide internet access and allow students time to research their assigned dairy product and answer the 2 questions on their handout. Encourage them to include detailed answers.
- When the groups have completed their research and recorded their answers on the handout, have them place their handout on the board. If their dairy product required a chemical change, place the paper on the side of the board labeled "Chemical Change." If the dairy product required a physical change, place the paper on the side of the board labeled "Physical Change" (from step 1).

- Go through each dairy product as a class to teach the remainder of the students how each product is made and what type of change (chemical or physical) takes place to make it. You may lead the discussion or have each team present the facts for their dairy product to the class. As students present, check for accuracy and correct as needed.
Elaborate
-
As students research their dairy product in Activity 2 (step 6), have them also find a short video clip to illustrate the process. If time permits, watch the video clips as a class to allow the students to actually see the process as it takes place in a milk processing plant. The "How It's Made" series produced by the Discovery Channel, SchoolTube, or YouTube are effective websites to search.
-
Though milk has been pasteurized and proven to significantly decrease the incidence of food-borne illness, some consumers would still like to consume raw, unpasteurized milk. Have students read the article found in the Essential Links, "Why is Milk Pasteurized? 4 Questions Answered." Challenge students to prepare a debate using scientific evidence to defend their stance.
-
Make cheese, ice cream, and/or butter in your classroom. Demonstrate a physical change by making butter and/or ice cream. Use the instructions found in the Better Butter activity to allow students to see cream turn into butter. Use the instructions found in the Ice Cream in a Bag activity to see milk turn into ice cream. Demonstrate the chemical change that takes place to make cheese. Use the Biotech Cheese Kit or visit www.cheesemaking.com for more instructions.
Sources
- Milk Pasteurization infographic provided by the National Dairy Council
- http://www.eatwisconsincheese.com/dairy/milk/milk-facts
- http://www.floridamilk.com/on-the-farm/florida-dairy-facts.stml
Recommended Companion Resources
- Better Butter
- Biotech Cheese Kit
- Casper Jaggi: Master Swiss Cheese Maker
- Dairy Tour 360
- Dairy in the Mountain West: Our Family of Farmers
- Food Chemistry Experiments
- Ice Cream in a Bag
- Louis Pasteur and Pasteurization
- Mozzarella Cheese Kit
- Say Cheese! A Kid's Guide to Cheese Making
- The American Dairy Industry