 Relevancy and Engagement
agclassroom.org/in/
Relevancy and Engagement
agclassroom.org/in/
Lesson Plan
FoodMASTER Middle: Fruits
Grade Level
Purpose
Students will learn the concept of enzymatic browning and methods for decreasing enzymatic oxidation by observing three types of fruit. Students will also understand the relationship between oxidation and antioxidants and the role fruits play in health and nutrition. Grades 6-8
Estimated Time
Materials Needed
Engage:
- Fruit: Where Does it Grow? slides or Fruit...Where Does It Grow? Kahoot
Lab 1 Teacher Materials:
- Paper plates and additional apples, bananas, and oranges for taste testing
- Paper towels, cutting board, and knife
Lab 1 Student Materials:
- Oxidation Transformation student handout, 1 per student (Key)
- Enzymatic Reactions lab sheet, 1 per student (Key)
Lab 1 Part A, per group of 4-5 students:
- 1/4 apple, 1/4 orange, 1/4 banana
- 1 cutting board
- 3 plastic or blunt knives (or apple corer)
- 3 paper plates
- 1 kitchen timer or stopwatch
- Paper towel
- Safety goggles
- Aprons (optional)
- 1 plastic sandwich bag if doing Part B on another day
Lab 1 Part B, per group of 4-5 students:
- 1 apple
- 2 - 9oz. plastic cups
- 1 cutting board
- Kitchen timer or stopwatch
- 1 paper plate
- 1 apple sample from "Part A"
- Safety goggles
- 2 household substances to test (chosen by group)
- 2 plastic spoons
- 1 plastic or blunt knife
- 1 beaker or measuring cup containing water
- 1/2 teaspoon measuring spoon
- 1 black permanent marker
- Aprons (optional)
- Household substances (Note that specific needed amounts will vary based on the number of groups that choose each substance.)
- Vitamin C tablets (1 tablet per group)
- Cream of tartar (1/2 tsp. per group)
- Lemon juice (1/2 cup per group)
- Sugar (1/2 tsp. per group)
- Baking soda (1/2 tbsp. per group)
- Vinegar (1/2 cup per group)
- Salt (1/2 tsp. per group)
Lab 2 Teacher Materials:
- Mortar and pestle (or spoon) for crushing vitamin C tablet
Lab 2 Student Materials, per group of 4-5 students:
- Hidden Antioxidants student lab sheet, 1 per student (Key)
- Safety goggles
- aprons (optional)
- 1 test substance chosen by group
- vitamin C tablets (1 tablet per group)
- cream of tartar (1/2 tbsp. per group)
- lemon juice (1/2 cup per group)
- vinegar (1/2 cup per group)
- 1 beaker or measuring cup containing water
- Important note: Colored iodine must be used in this experiment.
- 1 – 9 oz. plastic cup
- 1 plastic spoon
- 1 - 2 oz. cup containing colored iodine
- 1 medicine dropper
- 1 black permanent marker
Investigating Your Health Activity:
- Amazing Antioxidants student handout, 1 per student (Key)
- 3 food labels for a frozen, canned, and dried fruit.
- Food labels can be found in the grocery store, USDA’s FoodData Central, or by using the labels provided.
Essential Files
Vocabulary
antioxidant: a substance present in foods that can prevent or slow the harmful effects of free radicals in the body
enzymatic browning: the changes observed in fruits and vegetables caused by a reaction between enzymes and oxygen
enzyme: protein catalyst, which speeds up a specific chemical reaction
investigation control: the group or object in an investigation which is used as a comparison and undergoes no experimental treatment
oxidation: a chemical reaction between oxygen and other compounds
Did You Know?
- Apples store very well. Apples purchased at a store can be up to a year old.1
- A strawberry is not an actual berry, but a banana is.1
- Apples float in water because they are 25% air.1
Background Agricultural Connections
Fruits contain important disease-fighting compounds called antioxidants. Antioxidants aid in the prevention and repair of cells damaged from oxidation. Oxidation is a normal process that our body’s cells undergo, but it can cause stress on our bodies. It is important to eat foods containing antioxidants to help keep our bodies healthy and to treat and prevent the stress caused by oxidation. Antioxidants can be found in fruits, vegetables, grains, nuts, and spices. Fruits containing the highest concentrations are berries, such as cranberries, blueberries, and blackberries. Including these foods in our diets may help prevent diseases, including cancer and heart disease.

Vitamins and Antioxidants
Vitamins A, C, E, and the mineral zinc are antioxidants that can be found on the nutrition facts label. Vitamin C is the most common antioxidant, and it helps heal cuts and protect bones and teeth. Citrus fruits, including grapefruit, lemons, limes, oranges, and tangerines, are the highest in Vitamin C. Vitamin A is found in colorful fruits, like apricots and cantaloupe. Vitamin A helps your eyes. Vitamin E and zinc help your immune system and can be found in many different foods. The mineral selenium and the phytochemicals (see FoodMASTER Middle: Vegetables) lycopene, beta-carotene, and flavonoids are also antioxidants found in fruit.
Some of the antioxidants found in fruits (and some vegetables) have also been found to prevent other damaging processes, like enzymatic browning. Enzymatic browning occurs when enzymes catalyze the oxidation of phenols causing a food’s color to change to brown. Enzymatic browning can be both beneficial and detrimental for foods; however, it is not considered unhealthy to consume these foods after they have turned brown. For foods like tea and dried fruits, enzymatic browning is beneficial; it enhances their flavor and color. For foods like fresh fruits, vegetables and seafood, enzymatic browning is unfavorable because the brown color change causes them to appear aged.
Methods to Prevent Enzymatic Browning Reactions Application of Heat
- Application of Heat: Blanching - Fruits and vegetables are often boiled for 1-5 minutes (depending on size of produce) before freezing. Blanching inactivates enzymes by causing these proteins to denature and lose function.
- Sulfur Dioxide Dip: Sulfur dioxide is able to stabilize the color of fresh, and also processed fruits and vegetables. Sulfur dioxide stops the activity of oxidizing enzymes by removing the oxygen from the enzyme before pigments are formed. Sulfur dioxide also has antioxidant properties. It is often used in salad bars at restaurants to prevent unattractive browning.
- Sugar Syrup Dip: This method is one of the oldest to prevent browning. The sugar syrup coats the fruit and prevents the fruit surface from contacting oxygen.
- Vitamin C Dip: Vitamin C is a great antioxidant. It is oxidized instead of the catechol/tannin pigments that turn fruits brown when exposed to oxygen.
- Citric acid and acetic acid: Acids work to lower the pH of fruit tissue and reduce the action of polyphenol oxidase (browning enzyme). If the pH falls below 3.0 the enzyme will be inactivated.
FoodMASTER Middle Lessons
FoodMASTER (Food, Math and Science Teaching Enhancement Resource) is a compilation of programs aimed at using food as a tool to teach mathematics and science. For more information see the Background & Introduction to FoodMASTER for Middle School. This lesson is one in a series of lessons designed for middle school:
Engage
- Open the attached Fruit: Where Does it Grow? PowerPoint slides OR the Fruit...Where Does It Grow? Kahoot. Ask students to use their background knowledge to determine if each of the fruits grow on a tree, vine or bush.
- After the quiz, explain that most fruit grows on one of these three types of plants.
- Tree: Many trees produce various types of fruits and nuts. Fruit trees have a stem and branches made of wood. They produce flowers in the spring, which mature into fruit.
- Fruits that grow on trees: Lime, grapefruit, orange, apple, pear, cherry, peach, banana.
- Bush: A fruit bush is fairly low to the ground. It has small wooden stems that branch out. The bush is covered in leaves and the flowers mature into fruit.
- Fruits that grow on a bush: Pineapple, raspberry, and blueberry.
- Vine: Some fruits grow on vines. Vines such as those for grapes or kiwi fruits grow from a woody stem and are usually supported on a trellis. Watermelon and cantaloupe are examples of fruits that grow from vines with a soft, herbaceous stem.
- Fruits that grow on a vine: Grape, Strawberry, watermelon, and cantaloupe.
- Tree: Many trees produce various types of fruits and nuts. Fruit trees have a stem and branches made of wood. They produce flowers in the spring, which mature into fruit.
Explore and Explain
Lab 1: Enzymatic Reactions
Teacher Preparation:
- If possible, it is recommended that both Parts A and B of this lab be completed in one class period.
- Review information found in the Background Agricultural Connections section of the lesson, lesson Procedures, and the attached Essential Files.
- Prepare the following for each group:
- Part A: Cut thin slices for students to taste and place them on paper plates. In addition, slice apples, oranges, and bananas for the investigation in half. Each group will be given a 1/4 piece of each fruit type. If possible, this should be started at the beginning of the lesson so your students can observe the process.
- Tip: The extra apples are needed for the second lab in this lesson. It is important these apples remain in whole form until you are ready to begin the second lab to prevent enzymatic browning from occurring. Additionally, classroom temperature can affect how quickly the apple will brown. If your classroom is generally cold, oxidation effects may be less obvious. You may need to increase the amount of time students wait prior to observing a large change in the color of the apple’s flesh.
- Timesaver: Prepare fruit for the students ahead of time. The apples will be the only exception, as they should brown quickly. For quicker preparation of apples, use an apple corer. You may also consider assigning fewer fruits to each group and allow data to be shared. However, be sure to provide each group with one oxidizing fruit and one non-oxidizing fruit (e.g. apple and orange, or banana and orange). Be sure to adjust materials needed for this adaptation.
- Part B:
- Students will choose 2 treatments (e.g. vitamin C, baking soda, etc.) to include in their investigation. Organize materials in one location for easy distribution. Students will prepare their own solutions (if applicable). Have these substances available at different areas/stations in the room to maintain student flow and prevent crowding.
- Tip: If desired, some student groups may need to be asked to test certain substances to be sure all substances are tested.
- Timesaver: Prepare solutions ahead of time. Keep in mind each group will need to be able to test their prediction. This may require students to complete their predictions prior to the planned lab or the preparation of multiple samples for each possible substance.
- Students will choose 2 treatments (e.g. vitamin C, baking soda, etc.) to include in their investigation. Organize materials in one location for easy distribution. Students will prepare their own solutions (if applicable). Have these substances available at different areas/stations in the room to maintain student flow and prevent crowding.
- Part A: Cut thin slices for students to taste and place them on paper plates. In addition, slice apples, oranges, and bananas for the investigation in half. Each group will be given a 1/4 piece of each fruit type. If possible, this should be started at the beginning of the lesson so your students can observe the process.
Procedures:
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Enzymatic Reactions lab sheet and the Oxidation Transformation handout.
- Before beginning the lab investigation:
- Ask students to read Oxidation Transformation and complete the focus questions for this lab investigation.
- Require students to wash their hands.
- Allow students to taste a sample of apple, orange, and banana prior to beginning any investigation procedures. This process is important for increasing student exposure to healthy foods and decreasing the likelihood that students will be tempted to taste foods included as investigation materials.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances during the lab investigation.
- Show the Enzymatic Reactions Part A video to demonstrate the lab.
- Launch Part A of the lab by asking students to prepare their fruit. Students should then observe and make a prediction about their unknown substances. Instruct students to pay special attention to the timer as they collect their data. Students should see the following:
- Apple: Students should observe the apple’s flesh turning a brown color. As time passes, the brown color will become more distinct.
- Orange: Students will not observe any browning on the flesh of the orange.
- Banana: Students should observe browning of the banana slices. Browning will likely be less intense compared to the apples. The peel of the banana will also brown.
- Allow students to work in small groups on the Enzymatic Reactions lab sheet to further explore the topic and respond to lab questions.
- After completing the observation of enzymatic browning among three types of fruit and the investigation’s conclusions, students should be prepared to begin Part B. Be sure student groups set aside the apple sample from Part A of the lab investigation to serve as the control in Part B. If completing the lab over two class periods, store apple slices in sealed plastic bags.
- Distribute student lab materials for Part B with students organized into stations for easier distribution. Students should continue in groups of 4-5.
- Watch the Enzymatic Reactions Part B demonstration video.
- As a group, students should choose two substances they predict would prevent enzymatic oxidation.
- Students will prepare the chosen substances and test their prediction using an apple.
- Allow students to work in small groups on the Enzymatic Reactions lab sheet to further explore the topic and respond to lab questions.
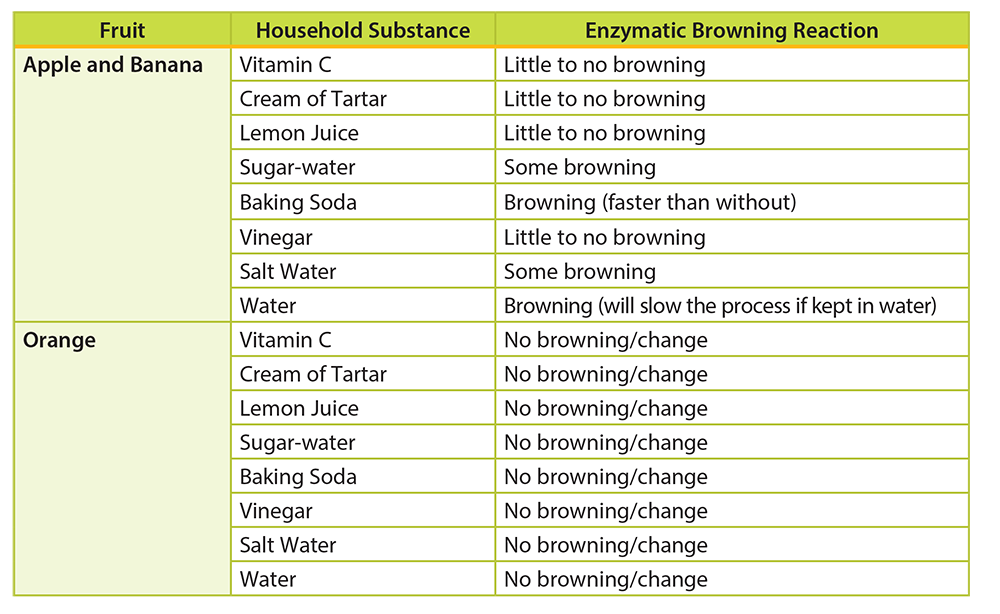
- Use the following information for a breakdown of expected reactions:
- Vitamin C: Little to no browning should occur due to the presence of vitamin C (antioxidant).
- Cream of Tartar: Little to no browning should occur. Cream of Tartar is an acid and will lower the pH of the fruit’s flesh.
- Lemon Juice: Little to no browning should occur due to the presence of vitamin C (antioxidant).
- Sugar-water: Some browning should be expected. Sugar water may coat the surface of the fruit’s flesh to prevent extensive browning.
- Baking Soda: Browning should be expected. Baking soda will increase the pH of the fruit’s flesh and hasten the browning effect.
- Vinegar: Little to no browning should occur. Vinegar is an acid and will lower the pH of the fruit’s flesh.
- Salt Water: Some browning should be expected. Salt water may coat the surface of the fruit’s flesh to prevent extensive browning.
- Water: Water can temporarily prevent browning if the fruit is submerged. It works by decreasing exposure of the fruit’s flesh to oxygen. If fruit is simply dipped in water, oxidation will occur. This sample can be used as an investigation control.
Lab 2: Hidden Antioxidants
Teacher Preparation:
- Review information found in the Background Agricultural Connections section of the lesson, lesson Procedures, and the attached Essential Files.
- Prepare materials for each group. Place approximately 20 drops of iodine into each 2 oz. or similar size cup. Set up stations from which students can obtain the test substance chosen.
- Tip: Groups may be assigned their test substance if it is desired that all substances be tested.
- Timesaver: Prepare solutions ahead of time. Keep in mind that each group will need to be able to test their prediction. This may require students to complete their predictions prior to the planned lab or the preparation of multiple samples for each possible substance.
- Tip: If sufficient amount of materials are not available, the testing of the substances may be done as a demonstration.
Procedures:
- Before beginning the lab investigation:
- Be sure students read Oxidation Transformation and completed the focus questions in lab one.
- Require students to wash their hands.
- Allow students to taste a sample of the food highlighted in the lesson prior to beginning any investigation procedures. This process is important for increasing student exposure to healthy foods and decreasing the likelihood that students will be tempted to taste foods included as investigation materials.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances used as part of the lab investigation.
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Hidden Antioxidants lab sheet.
- Launch the lab by asking students to make a prediction about the antioxidant potential of various acidic substances.
- Students should choose one substance to test their prediction. After obtaining and recording observational data within each group, allow the groups to share their findings with the class. This step is necessary for each student to complete Table A.
- Tip: Groups may be assigned their test substance if it is desired that all substances be tested.
- Refer to the following information for a list of expected reactions:
- Cream of Tartar: Iodine should change the color of the solution to an opaque golden yellow (pH ~ 4).
- Vinegar: Iodine should change the color of the solution to a medium clear, dark, golden yellow (pH ~ 3).
- Vitamin C: Iodine should disappear when added to the solution. The solution should remain clear. However, if iodine is continually added, eventually the iodine will completely oxidize the vitamin C present, potentially resulting in a color change (pH ~ 3, slightly lower than vinegar).
- Lemon Juice: Iodine should disappear when added to the solution. The solution should remain its original color. However, if iodine is continually added, eventually the iodine will completely oxidize the vitamin C present, potentially resulting in a color change (pH ~ 2).
- Show students the video lab demonstration, Part II: Hidden Antioxidants.
- Iodine can be used to detect vitamin C. When iodine is added to a vitamin C containing solution, ascorbic acid (vitamin C) is oxidized to dehydroascorbic acid and iodine is reduced to iodide ions.
- C6H8O6 (Ascorbic acid) + I2 (Iodine) → 2 I- (Iodide) + C6H6O6 (dehydroascorbic acid)
- Vitamin C tablets and lemon juice are the two substances with antioxidant properties. Vitamin C is an antioxidant and is contained in both substances. Oranges are also high in Vitamin C. In Part I-B of this lab, students should have observed the vitamin C solution and lemon juice prevent oxidative browning in apples. Other substances (e.g. sugar) can prevent oxidation browning; however, they work through other mechanisms such as reducing exposure of the fruit’s flesh to oxygen or lowering the pH.
- Allow students to work in small groups to complete the Hidden Antioxidants lab sheet.
- Follow-up with a class discussion about antioxidants and their role in human health.
Investigating Your Health: Amazing Antioxidants
- Give each student one copy of the Amazing Antioxidants handout.
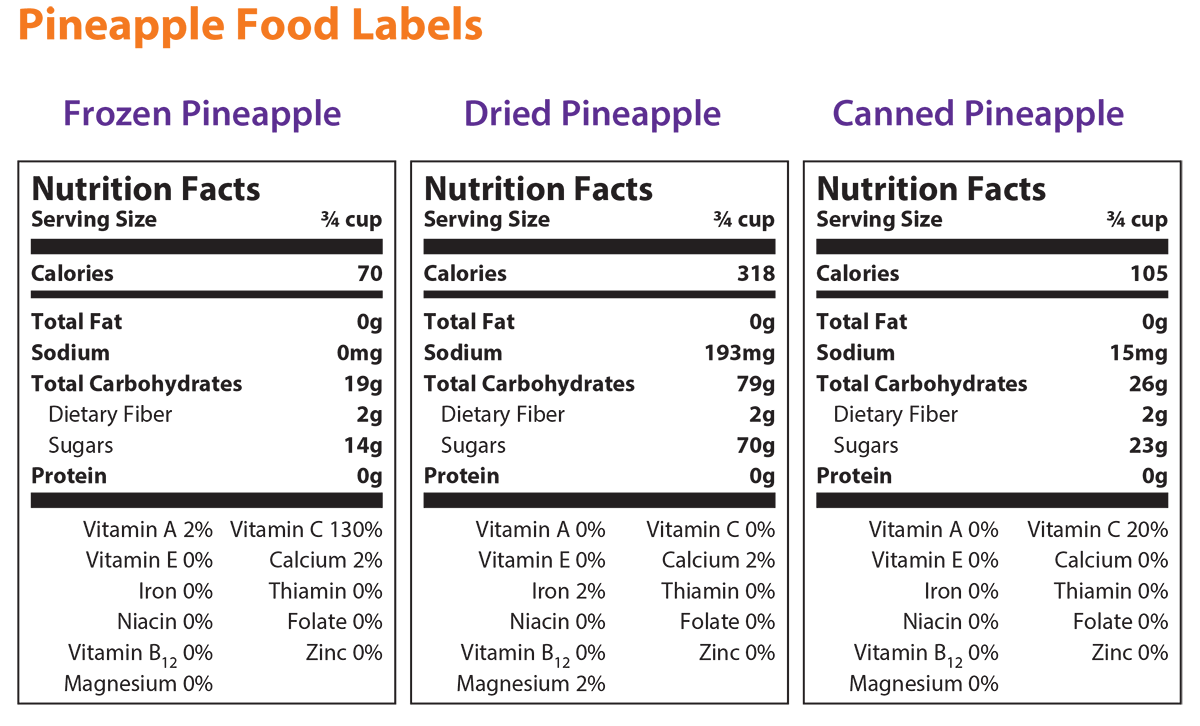
- Using the information provided in the Background Agricultural Connections section and/or information learned from researching antioxidants, students should examine the food labels for a single fruit packaged in three different ways (i.e. frozen, dried, and canned).
- Students can find food labels in the grocery store, USDA’s FoodData Central, or use the labels provided.
- If you choose to use the provided pineapple food labels, see the attached teacher key for answers to the Investigating Your Health lab questions. Answers to questions based on other food labels will vary.
- This assignment can be completed in-class or as homework. If completed in-class, allow students to work in small groups on the Investigation worksheet to further explore the topic and respond to questions.
- Follow-up with a class discussion about student findings related to the health benefits of fruits and student generated ideas for increasing fruit consumption.
Elaborate
-
Students can take photos of the fruit at different time intervals and create a poster explaining the investigation.
-
Explore enzymatic browning in different varieties of apples (e.g. Granny Smith versus Red Delicious).
-
Students can create or bring in recipes that include antioxidant-containing fruit as a primary ingredient (e.g. fruit salad with lemon juice as ingredient).
-
Explore other methods to prevent enzymatic browning. An acid with a lower pH is the key. Name other fruits that have a low and high pH. Test your theories by setting up and performing an investigation.
-
Test the antioxidant potential of oranges by setting up another iodine investigation.
Acknowledgements
This lesson was partnered with East Carolina University. The FoodMASTER program was supported by the Science Education Partnership Award (SEPA) which is funded from the National Center for Research Resources, a component of the National Institutes of Health.
- Primary Authors:
- Virginia Stage, PhD, RDN, LDN
- Mary White
- Ashley Roseno, MAEd, MS, RDN, LDN
- Melani W. Duffrin, PhD, RDN, LDN
- Graphic Design: Cara Cairns Design, LLC